CLS-014
CLS-014 is the first gene therapy drug designed to destroy circulating cancer cfDNA
CLS-014 is a preclinical stage, first-in-class gene therapy platform technology for the treatment of different gastrointestinal malignancies, utilizing a novel anti-cancer therapeutic target: cell-free DNA.
Elevated level of cell-free DNA is a hallmark of different tumors and is a previously overlooked trigger implicated in driving the cancerogenesis, metastasis and chemotherapy resistance.
We are developing CLS-014 intended to directly address this problem by destructing blood cell-free DNA in cancer patients. Our engineered adeno-associated viral vector technology is designed to achieve efficient gene-expression of the DNase I enzyme in the liver leading to its secretion into the systemic circulation to effectively target and destroy blood cfDNA.
We know that CLS-014 holds promise for the treatment of different tumors, since elevated cfDNA is implicated in driving of a variety of malignancies, starting with pancreatic adenocarcinoma, one of the deadliest cancers.
Pancreatic, liver, and stomach cancers are characterized by elevated levels of cfDNA, which plays a crucial role in their progression.
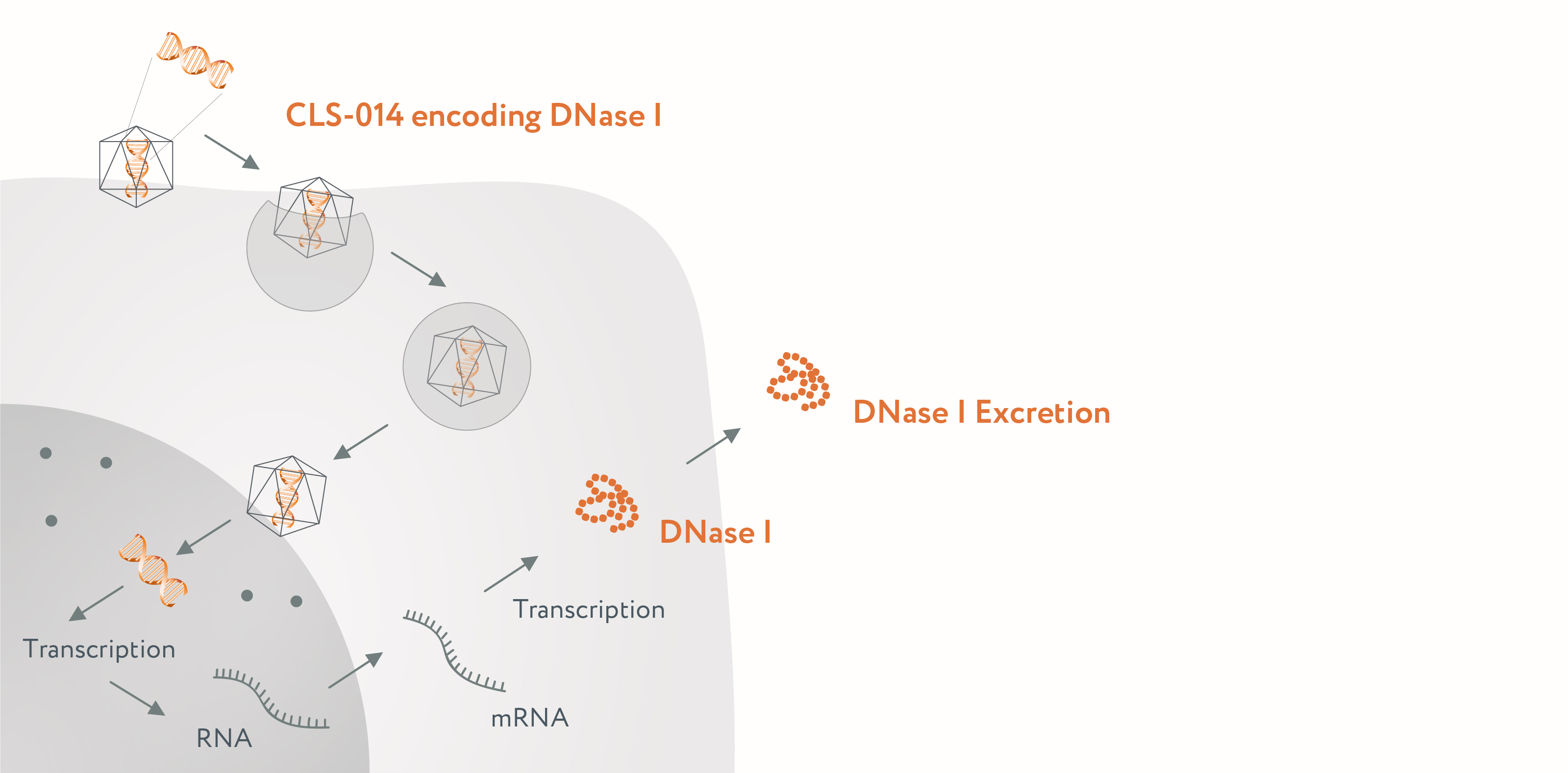 Upon a single intravenous injection, CLS-014 transduces liver cells and induces them to synthetize and secrete a hyperactive non-immunogenic variant of human DNase I. This DNase I is released to a systemic circulation where it cleaves all types of elevated cfDNA associated with tumor progression.
Upon a single intravenous injection, CLS-014 transduces liver cells and induces them to synthetize and secrete a hyperactive non-immunogenic variant of human DNase I. This DNase I is released to a systemic circulation where it cleaves all types of elevated cfDNA associated with tumor progression.
CLS-014 has been designed to work as a double-edged sword being the adjunctive treatment to any first-line chemotherpay through suppression of tumor growth and metastasis development as well as prevention of the devastating effects of circulating cfDNA on patients’ immune, cardiovascular and central nervous systems.
First and foremost, CLS therapeutics is developing CLS-014 for the therapy of advanced pancreatic adenocarcinoma that remains the deadliest major cancer in the US.
Along with the efficacy for the treatment of pancreatic cancer, CLS-014 has the potential for multiple follow-up indication extensions including treatment of various cancers of gastrointestinal origin characterized by elevated cfDNA levels.